Digitally Enhanced Healthcare Solutions (1)One Hitachi Approach to Combining OT and IT Systems in Single Package towards Digitalization of Next-generation Modular Cell Processing Centers
Highlight
Against a background of rapid advances in biotechnology along with the longer healthy life spans and rising healthcare costs associated with aging populations, recent years have seen rising demand for advanced diagnostic techniques, control of healthcare costs, and greater use of personalized medicine.
This series of articles focuses on advanced healthcare solutions powered by Hitachi’s digital technologies, describing the work being done to realize a “wellbeing society” and the plans Hitachi has for this field in the future.
Traceability and measures for preventing patients’ cells from getting mixed up are essential for patient safety at the sterile pharmaceutical manufacturing facilities that produce drugs free of bacterial contamination and in CPCs that produce cultured cell products like those used in regenerative medicine. To meet these needs, management practices can be strengthened through the use of systems that link patients, medical institutions, and CPCs together. This article describes initiatives for building advanced healthcare solutions that have been made possible by the development of control boards (modules) for equipment digitalization, especially in CPCs.
By providing efficient maintenance management and the environments for cell culturing and processing that customers are actually looking for, Hitachi is working to create a society in which patients who require regenerative medicine can receive effective treatments that are safe and secure.
1. Introduction
Conventional medicines are made from low-molecular weight compounds or cellular secretions. Recent years, however, have seen considerable research into regenerative medicine, whereby cells are taken from the body, manipulated, and then used to treat the disease from which the patient suffers. There are high hopes for its use in practice.
The cells used in regenerative medicine are mainly processed in cell processing centers (CPCs). CPCs are split up into separate zones that house the different processes, with each of these aseptic clean rooms being maintained at a high level of cleanliness in accordance with their respective criteria. A feature of such facilities is that air cleanliness and zone air pressures are managed on the basis of predefined grades (see Table 1).
Table 1—Maximum Permitted Airborne Particle Concentration for Different Manufacturing Area Grades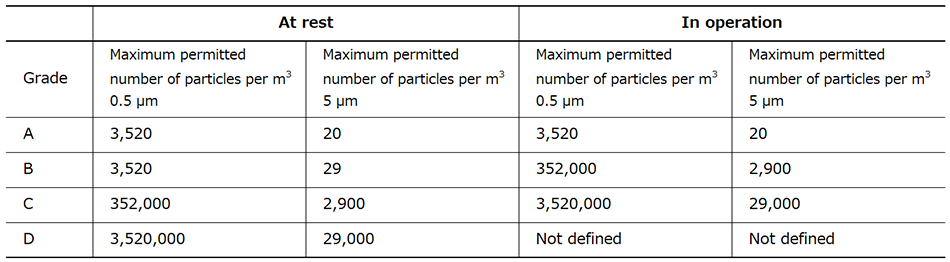 Source: WHO Technical Report Series, No. 961, Annex 6 (2011) Cleanliness must satisfy the criteria both when the plant is operating and when it is at rest.
Source: WHO Technical Report Series, No. 961, Annex 6 (2011) Cleanliness must satisfy the criteria both when the plant is operating and when it is at rest.
CPCs typically use ducts to control the temperature, humidity, air pressure, and cleanliness of their respective zones. The amount of space taken up by ducting has increased as structural designs have become more complex, and this has lengthened construction times. By moving away from past practices and instead using fan filter units (FFUs) for non-ducted, in-ceiling installation, Hitachi Global Life Solutions, Inc. developed a next-generation modular CPC in 2019 that can maintain each zone’s air pressure and cleanliness. These CPCs have been supplied to customers in Japan and elsewhere.
2. Challenges for CPCs and Use of IoT-CPCs as a Solution
In the field of regenerative medicine, getting cells mixed up can result in medical accidents. This makes it important to keep track of information all the way from when the patient’s cells are harvested until the resulting products are administered. Improving the traceability of cell processing is a crucial challenge for the value chain.
While the job of cell culturing at a CPC includes many intricate steps that call for a high level of concentration by staff, workforce shortages mean that, in many cases, the people engaged in this work also have other duties to perform. To maintain the quality assurance and productivity of cell culturing, it helps to create an environment in which these staff can concentrate on the work of culturing itself.
Hitachi is already using its integrated management platform, the Hitachi Value Chain Traceability Service for Regenerative Medicine (HVCT RM)(1), to ensure traceability by delivering services for coordinating information across the different parties involved on a common cloud platform. This includes cell processing providers, transportation services, and medical institutions. Similarly, it also uses the Hitachi Pharmaceutical Manufacturing Execution System (HITPHAMS)(2) for production and quality management. This system handles instructions and record keeping for each step in the cell culturing process at CPCs to help improve manufacturing efficiency and to provide the strict management stipulated by regulation.
To overcome the challenges facing CPCs, Hitachi is working on making it easier to keep track of the cell culturing process by installing operational technology (OT) and IT systems at CPCs (see Figure 1).
Figure 1—CPC Block Diagram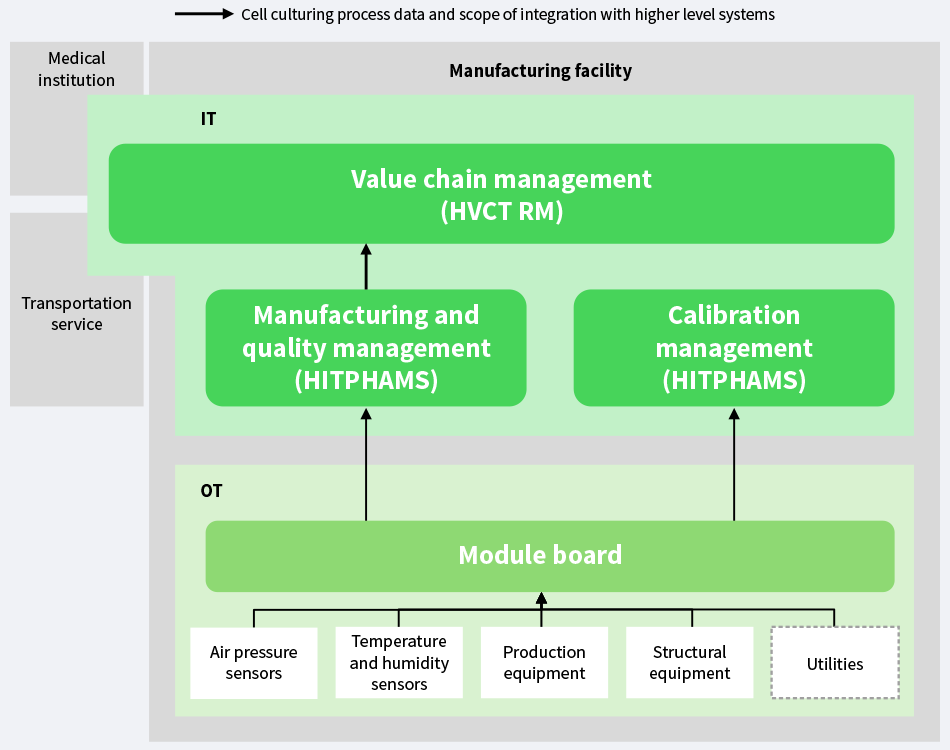 HVCT RM: Hitachi Value Chain Traceability Service for Regenerative Medicine
HVCT RM: Hitachi Value Chain Traceability Service for Regenerative Medicine
HITPHAMS: Hitachi Pharmaceutical Manufacturing Execution System
OT: operational technology The diagram shows the data flows between the CPC and other systems.
2.1 Development of Module Boards as OT Systems
Air conditioning control works by outputting signals to the air conditioning systems to keep them within the required operating range. This is done by connecting the temperature and humidity sensors to an indicator controller and performing proportional-integral-differential (PID) control. The FFU control used to maintain cleanliness regulates the air pressure by inputting an air pressure signal from the indicator controller to the programmable logic controller and implementing FFU speed control in the PLC program.
Common practice for passing data on temperature, humidity, air pressure, and cleanliness and from the air conditioning system and FFUs to the IT system is to perform this data collection on a separate PLC and supervisory control and data acquisition (SCADA) system. This handles communication between the equipment and IT systems. To integrate this into a single package, Hitachi consolidated the indicator controllers and PLC control functions, developing a new module board incorporating an interface for communicating with IT systems (see Figure 2). The expansion capabilities of the IT system communication function allow for a simpler system configuration for traceability management. This represents an early implementation of the Internet of Things (IoT) in a CPC (referred to here as “IoT-CPC”), thereby helping to provide patients with safe and secure treatment.
Figure 2—Comparison of Module Board with Previous System 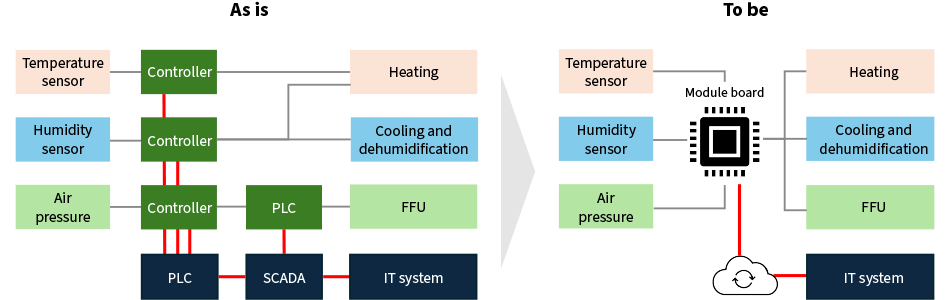 PLC: programmable logic controller
PLC: programmable logic controller
FFU: fan filter unit
SCADA: supervisory control and data acquisitionThe diagram shows the scope of control by the module board for air conditioning and air pressure control and the connection to the data cloud.
2.2 Seamless Operation Achieved through IT System Interoperation (HITPHAMS and HVCT RM)
HITPHAMS is a package system that supports every step in the cell culturing process at CPCs, from the arrival of patient cells to product delivery. It includes a function for tracking information on the materials used on cells and a cross-checking function that prevents cells from getting mixed up. To incorporate this into a single CPC-integrated package, a standard interface for communication between HVCT RM and HITPHAMS was developed in parallel with the module board (shown in red in Figure 2). This establishes bi-directional links between cell records and allows for seamless operation across the different parties involved, helping to ensure safety and delivering end-to-end (E2E) traceability from patient cell harvesting to when the resulting products are administered. This also helps to reduce operational workloads as both HITPHAMS and HVCT RM are cloud services supplied in software-as-a-service (SaaS) form.
2.3 Improved CPC Efficiency from Digitalization of Periodic Validation
To maintain process quality at CPCs, validation is performed by taking measurements of temperature, humidity, air flow, air pressure, cleanliness, and other parameters using calibrated instruments. This is done to verify the performance of air conditioning control and to ensure that each zone satisfies the grade it was designed to achieve. Given the risk of drift in equipment performance over time, this validation is performed periodically to maintain the designed performance.
As periodic validation requires expertise in the equipment concerned, Hitachi Global Life Solutions offers it as a service. Unfortunately, this validation takes a lot of work, requiring multiple personnel who need to be on-site at the same time to allow for operation of the relevant equipment and the back-and-forth communication of measurements from control panels or monitors located in different rooms. As cell culturing at the CPC needs to halt while this periodic validation is in progress, it also helps if the work can be completed quickly.
Accordingly, Hitachi has established a new operating practice that uses the calibration management function in HITPHAMS referred to above. Measurements from the air conditioning equipment are collected by the module board and forwarded to HITPHAMS for archiving. By doing the work digitally, this has the potential to make validation more efficient, allowing it to be completed using fewer people than were required in the past while at the same time improving work quality. It is anticipated that it will shorten the time required for equipment calibration by about 30%. By performing periodic validation more quickly, this should improve CPC utilization and thereby hasten the delivery of treatments to patients.
Figure 3—Comparison of Periodic Validation Work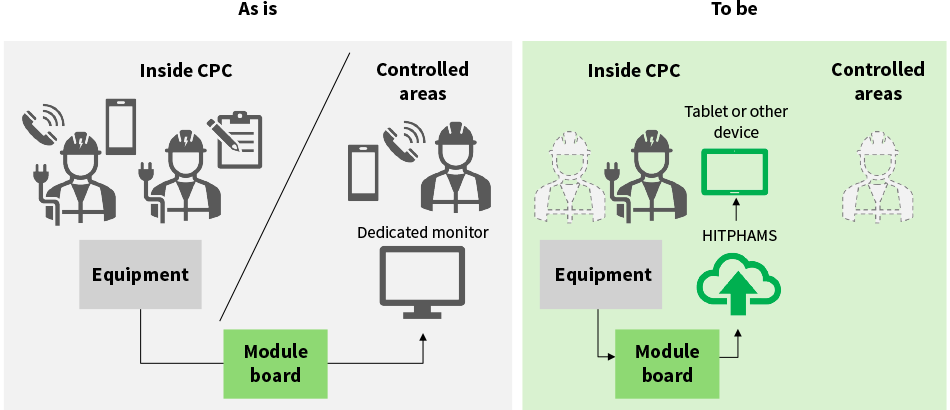 This shows an example of specific changes in periodic validation (calibration).
This shows an example of specific changes in periodic validation (calibration).
3. Conclusions
This article has described an upgrade to modular CPCs incorporating OT and IT systems. Hitachi is currently working on the development of new services that utilize the data accumulated in IoT-CPCs.
In the future, Hitachi intends to strengthen its connections with corporate partners with a view to deploying technologies for regenerative medicine beyond Japan and to persevere with technology development so that it can go on delivering solutions that contribute to societies globally.



