Digitally Enhanced Healthcare Solutions (2)Digital Solutions for Regenerative Medicine Supporting Process Development, Production Management, and Quality Management
Highlight
Regenerative medicine is a technique for utilizing the inherent functions of human cells to restore functions lost due to injury or illness. It is recognized for its potential as a new form of therapy that can help to improve patients’ QoL. The use of human cells as raw materials, however, means that establishing manufacturing practices that ensure the quality, efficacy, and safety of its medical products poses a variety of challenges.
Hitachi is working to establish regenerative medicine as an industry and to expand the market, drawing on its strengths in OT and IT to overcome the challenges at every step along the engineering chain by supporting objective science-based decision-making. This includes seamlessly integrating the data associated with cell product quality across all steps from research and development to routine manufacturing.
This article describes digital solutions for regenerative medicine that support process development, production management, and quality management.
1. Introduction
Nations around the world, including Japan, are facing aging populations and this along with a growing focus on health is driving demand for medical products with high levels of safety and efficacy. Keeping healthcare costs under control through effective treatment and drug administration is another major challenge for society. Meanwhile, with the goal of delivering wellbeing across all areas of society, high hopes are being placed on personalized medicine and the associated improvements in patient quality of life (QoL). Personalized medicine tailors health management to individual patients and enhances the effectiveness of therapies.
One area in particular that is recognized as having the potential to help improve patient QoL is regenerative medicine. Regenerative medicine works by using human cells and tissues as raw materials and the hope is that it will offer new therapies and medical products that can restore functions lost due to injury or illness. In Japan, the field has moved on from its pioneering days to enter a growth phase, with 20 regenerative medicine products having received approval as of April 2024. This was prompted by the passing in 2014 of the Pharmaceutical and Medical Device Act (PMD Act)*1, which defined regenerative medicine products separately from other pharmaceuticals and medical devices.
As regenerative medicine uses human cells and tissues as raw materials, however, it faces a variety of challenges when it comes to establishing manufacturing practices that ensure the quality, efficacy, and safety of its medical products. One example is that having cells both as an input and present in the end product means that heat and other forms of sterilization cannot be used. Instead, the aseptic condition of the product needs to be guaranteed at every step along the manufacturing process, a requirement that imposes high costs. Similarly, with manufacturing practices still evolving, it is frequently the case that there is uncertainty about the feedstock cells and parameters of the manufacturing process that impact on product quality. This makes it difficult to optimize the manufacturing process based on the scale of operation or to suit specific production facilities while still maintaining quality. Meanwhile, when it comes to information about the materials used in manufacturing and the clinical performance of products at medical facilities, the standardization, sharing, and use of data remains a work in progress.
Hitachi is drawing on its strengths in operational and information technologies (OT and IT) to support objective science-based decision-making, providing seamless integration of the data associated with cell product quality across all steps from research and development to routine manufacturing. By doing so, it is working to establish regenerative medicine as an industry and to expand the market by overcoming the challenges at every step along the engineering chain (see Figure 1).
This article describes digital solutions for regenerative medicine that support process development, production management, and quality management. This includes reviewing supporting technologies for contamination control strategies in cell manufacturing, supporting technologies for manufacturing processes, and initiatives for establishing an ecosystem, and looking at future developments.
- *1
- Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices
Figure 1—Engineering Chain for Cell Products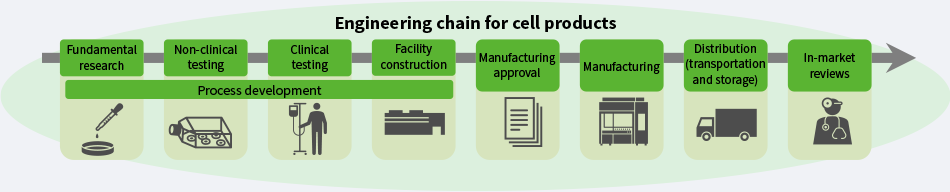 In addition to supporting objective science-based decision-making based on the seamless integration of data associated with cell product quality across all steps from research and development to routine manufacturing, Hitachi is also playing its part in expanding the market for regenerative medicine and getting it established as an industry by overcoming the challenges at every step along the engineering chain.
In addition to supporting objective science-based decision-making based on the seamless integration of data associated with cell product quality across all steps from research and development to routine manufacturing, Hitachi is also playing its part in expanding the market for regenerative medicine and getting it established as an industry by overcoming the challenges at every step along the engineering chain.
2. Supporting Technologies for Contamination Control Strategies in Cell Manufacturing
Businesses that manufacture regenerative medicine products and sterile pharmaceuticals need to comply with the regulatory requirements of Japan and other jurisdictions while at the same time ensuring product quality, reducing production costs, and improving production efficiency. In particular, there is a requirement to formulate a contamination control strategy (CCS) that addresses risks relating to the prevention of cross-contamination or contamination by foreign matter, as defined in Annex 1 of the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S). This calls for comprehensive contamination control that deals with both the “hard” and “soft” aspects, namely manufacturing equipment on the one hand and operating procedures and training on the other. Logical explanations of the CCS for the entire facility and data that can serve as evidence are both important parts of this process. Despite this, in many cases, risk assessment and environmental data evaluation still often depend on who is performing the work.
2.1 CCS Support Service
To address these issues, Hitachi has developed and implemented tools that provide manufacturers with the data they need for CCS activities and has made them available as services. These digital services continuously collect the information and evidence needed for contamination control across all steps along the engineering chain, from process development by business operators to manufacturing, and uses it for analysis (see Figure 2). One such service is a hygiene control analysis support tool that provides the evidence data for hygiene control, an aspect of the operation that has a strong bearing on facility utilization, and for optimizing operations at the facility. The data made available by this tool provides a facility-wide view of operations and shows the environmental conditions in manufacturing, enabling the manufacturer to make improvements that boost facility utilization.
In addition to making these tools available individually, Hitachi also plans to integrate them with other tools and systems to offer extended services that can benefit all areas of quality management. This includes the work going into functional enhancements that support all aspects of lifecycle management from strategy formulation to execution management, evaluation, and improvement. One example of this is data integration with a manufacturing management system that seeks to optimize facility utilization so that hygiene control can be done in a way that takes account of the manufacturing schedule. Future plans include the progressive roll-out of functions that support the management of CCSs while also further expanding the scope of system interoperation and data acquisition. Through these services, Hitachi will supply the data that manufacturers need for CCSs and help to achieve both quality assurance and cost reduction.
Figure 2—How CCS Support Services are Used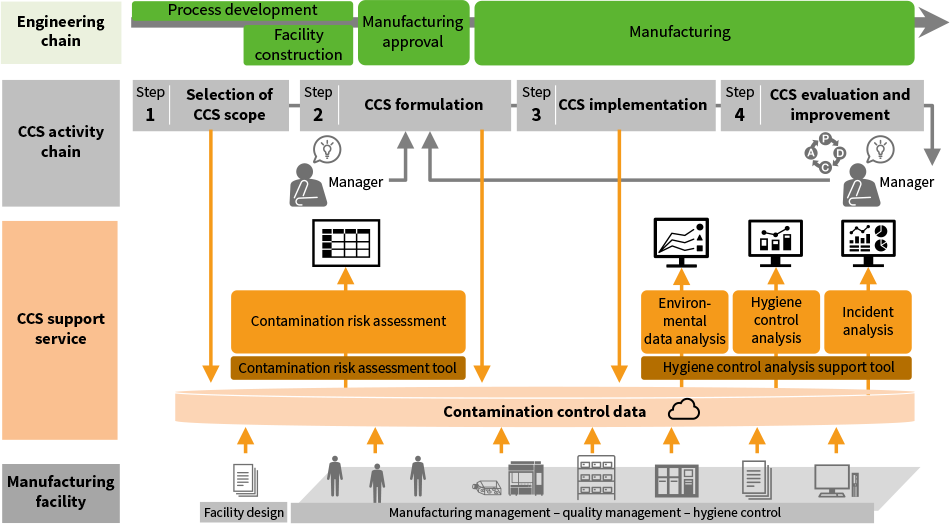 CCS: contamination control strategyThese services give access to inter-linked information and data that relates to CCS activities and provide the results of analysis as feedback. They have uses at all steps in the CCS activity chain that is undertaken along the engineering chain.
CCS: contamination control strategyThese services give access to inter-linked information and data that relates to CCS activities and provide the results of analysis as feedback. They have uses at all steps in the CCS activity chain that is undertaken along the engineering chain.
2.2 Demonstration Project for CCS Support Tool
Hitachi commenced joint research on hygiene control for cell manufacturing facilities with the CiRA Foundation of Kyoto University in July 2022. Based on this work, a demonstration project is underway at the Yanai Facility for my iPS Cell Therapy that the CiRA Foundation plans to start using from April 2025, making use of the CCS support tools developed by Hitachi. Through its iPS Cell Stock Project and my iPS Project, the CiRA Foundation plans to provide clinical induced pluripotent stem (iPS) cells manufactured from patients’ own cells to research institutions and other companies for about one million yen. To this end, it is working with a number of companies on the development of an enclosed automatic culture system with the goal of reducing manufacturing costs. Hitachi is supporting the my iPS Project, utilizing its tools for data application to facilitate operational optimization of the cell manufacturing area at the Yanai Facility for my iPS Cell Therapy while also helping to achieve both quality assurance and cost reduction.
3. Supporting Technologies for Manufacturing Processes
Because regenerative medicine uses live cells with ever-changing quality characteristics as raw materials, and because these cells are present in its end products, it is vital that the manufacturing process be put together in such a way that quality is maintained at every step. Hitachi is developing enabling technologies for manufacturing process development that draw on its Lumada*2 data analysis techniques and on technologies and know-how acquired from supplying the pharmaceuticals industry with OT and IT systems. These have included manufacturing and quality management systems along with culture systems and other production equipment and machinery.
- *2
- Hitachi’s advanced digital solutions, services, and technologies for turning data into insights to drive digital innovation
3.1 Simulation of Cell Manufacturing
Cell manufacturing for regenerative medicine involves seeding a culture vessel with, for example, on the order of 106 cells and then culturing them. Because the condition of the individual cells is not uniform and is also affected by the culture conditions and the incubation process, which includes tasks like seeding and replacing the culture medium, a lot of experience is needed to determine the optimal parameters at the process development stage. To overcome this challenge, Hitachi is developing simulation techniques for modeling of the incubation process and cell behaviors associated with non-uniformity to predict how process parameters such as the cell count and rate of growth will change(1). A feature of the simulation is that it considers three different energy balances, namely the cell-cell adhesion energy, cell-substrate adhesion energy, and cell migration energy. It uses this to replicate the behavior of individual cells based on characteristic values that relate to cell division and adhesion, thereby being able to predict the collective condition of the cells in a way that considers interactions with nearby cells.
3.2 Overview of Simulation Platform for Cell Manufacturing
The simulation platform for cell manufacturing utilizes these technologies and is equipped with cell simulation that models the culture and other processes performed in cell manufacturing, analysis tools for viewing and comparing the results of multiple simulation runs using different conditions, and a service application programming interface (API) for interoperation with other systems. By enabling the use of simulation as an alternative to using cells for experimental testing and as a way to identify factors that influence the quality of cell manufacturing, these features enable process development to be accomplished with fewer experimental runs. In doing so, the platform helps to achieve reliable cell quality and to make process development more efficient (see Figure 3).
In the future, Hitachi intends to work with Osaka University and corporate partners to verify the utility of simulation practices and to establish solutions for manufacturing process development.
Figure 3—Block Diagram of Simulation Platform for Cell Manufacturing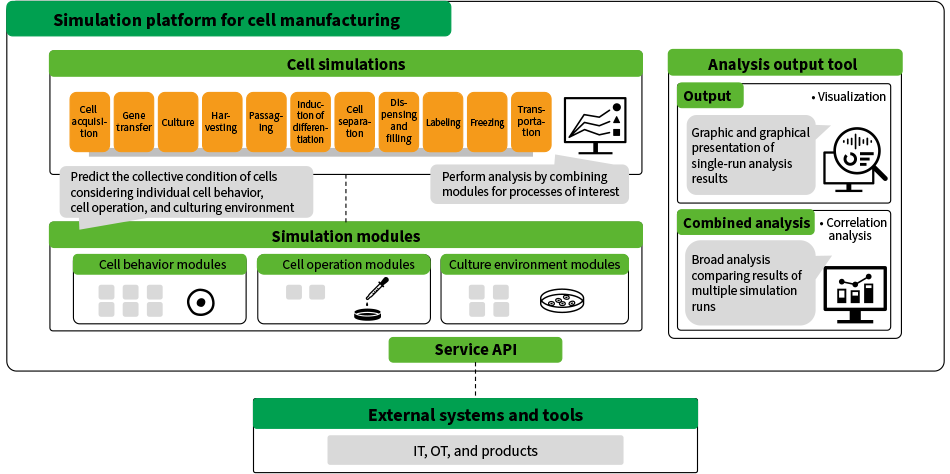 API: application programming interface, OT: operational technologyThe platform combines separate simulation modules for each of the manufacturing processes to model cell behavior in the processes concerned. Through its use to analyze the simulation results and identify the optimal manufacturing conditions, the platform serves as a solution for manufacturing development.
API: application programming interface, OT: operational technologyThe platform combines separate simulation modules for each of the manufacturing processes to model cell behavior in the processes concerned. Through its use to analyze the simulation results and identify the optimal manufacturing conditions, the platform serves as a solution for manufacturing development.
4. Initiatives for Establishing Ecosystem
A number of biotech clusters have been established as part of efforts to establish the regenerative medicine industry in Japan. These include the Kobe Biomedical Innovation Cluster and the King Skyfront, Tonomachi International Strategic Zone. The International Center for Future Medicine Nakanoshima Qross*3 was established in Nakanoshima, Osaka in 2024 and features involvement by a wide range of organizations. These include academia and start-up companies in addition to the medical institutions that harvest cells from patients and provide treatment, the pharmaceutical companies that handle cell manufacturing, and the suppliers who provide the equipment and consumables.
Through its supply of IT systems, Hitachi has been working to build an ecosystem for getting future healthcare practices established, having participated in the Organization for Advanced Healthcare Innovation, the entity that operates Nakanoshima Qross, since 2019 and being numbered among its founders.
- *3
- Nakanoshima Qross is a registered trademark in Japan of the Organization for Advanced Healthcare Innovation.
4.1 Collaboration with Stakeholders
Through its participation in the Organization for Advanced Healthcare Innovation, Hitachi has identified a number of issues posed by industry development that relate to stakeholders working together. One is that regenerative medicine product development has a need for culture flasks, centrifuge tubes, and other such items used in a cell culture. As different phases, such as research and development or clinical testing, have different requirements for these items in terms of their level of sterility and so on, product selection has proven time-consuming. To shorten the time this takes, there has been sharing of performance and other details about the parts and materials belonging to the corporate participants in the organization.
The next issue arises because the nature of regenerative medicine is such that live human cells are used in therapies. Accordingly, medical institutions that adopt these therapies need to provide additional training to staff on aseptic handling practices. They also need to apply for the associated approvals. While collecting the necessary information at a busy medical institution can be an obstacle to adoption, this can be addressed by matching these institutions with partners such as consulting services or organizations that offer assorted training programs.
When processed and dispatched as a product, human cells need to be transported and stored under appropriate temperature-controlled conditions. The matching of users with appropriate temperature-controlled storage or transportation services is an effective means for the timely supply of product.
Through the communications and matching support provided by the activities of the Organization for Advanced Healthcare Innovation, Hitachi has established IT systems that address these issues by enabling the selection of appropriate parts and materials, the collection of information on training programs, and the selection of storage and transportation services in ways that are faster and more efficient. Hitachi intends to continue paying close attention to user needs as it works to establish regenerative medicine as a viable industry.
4.2 Partnership between Healthcare and Manufacturing
Hitachi is also building information systems that collect clinical data from the medical institutions participating in the Organization for Advanced Healthcare Innovation and manufacturing data held by the companies, utilizing it as feedback for work on quality verification and improvement or research into developing new product generations. The goal for the future is to establish schemes that use these systems for the evidence-based assessment and verification of safety and efficacy.
5. Conclusions
This article has described techniques that use digital technology as a platform to support development and manufacturing for regenerative medicine. It has also described the work going into establishing an ecosystem. Through these efforts, Hitachi intends to continue making its contribution to the growth of customer businesses by serving as an industry partner for regenerative medicine and through the development and supply of digital solutions as “One Hitachi,” accelerating the work being done with GlobalLogic and Hitachi’s Connective Industries Sector that has products and solutions for the field of regenerative medicine.
Acknowledgements
The activities described in this article benefitted from the assistance of the CiRA Foundation, Kyoto University (supporting technologies for contamination control strategies in cell manufacturing), the Graduate School of Engineering at Osaka University (supporting technologies for manufacturing processes), and the Organization for Advanced Healthcare Innovation (initiatives for establishing an ecosystem). The authors would like to take this opportunity to express their deepest gratitude.



